-
Hello guest! Are you an Apistogramma enthusiast? If so we invite you to join our community and see what it has to offer. Our site is specifically designed for you and it's a great place for Apisto enthusiasts to meet online. Once you join you'll be able to post messages, upload pictures of your fish and tanks and have a great time with other Apisto enthusiasts. Sign up today!
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What happens to ph when you mix ro water with tap water ?
- Thread starter anewbie
- Start date
- Messages
- 648
- Location
- San Francisco
I understand that pH isn't stable at low conductivity. But I don't know what to make of observed values for keeping and breeding the true blackwater species. i.e., Low conductivity with specific recommendations for low pH. Are these pH recommendations, therefore, meaningless?I didn't choose the conductivity datum for any reason other than that is about the highest conductivity value that I ever find in the rain-water I use for the tanks. If I had had softer rain-water? I would have used a lower conductivity datum and it would open up the possibility of keeping (and even breeding) more extreme black water fish.
If I start with more alkaline water, I can observe that peat acidifies that water much more effectively than other botanicals (alder cones, leaves of various kinds). Indeed, many aquarists who've bred blackwater species have used RO water and acidify with peat.
If I want to breed blackwater species but I don't want to use peat, is it just the low conductivity that matters, or do I need to care about how many proton donors I'm adding to the water? For example, if I add tannins with a more neutral botanical like coir, should I also add a weak organic acid? Should I consider more acidic botanicals like redwood bark?
Assume that I will start with RO water and remineralize. I probably don't want to start with my tap, which is soft, but contains NaOH.
Thanks
- Messages
- 2,868
- Location
- Wiltshire UK
Hi all,
Sphagnum peat works via <"ion exchange">, <"active substrates"> (that reduce pH) work in the same way.
cheers Darrel
No, you just have to bear in mind that pH is more and more unstable as you approach pure H2O. Natural black water will remain acidic. A floating plant (or tree in the flooded forest) would have less effect on pH, because the oxygen evolved will mainly enter the atmosphere, rather than into the water.Are these pH recommendations, therefore, meaningless?
Acids will add protons (H+ ions) and these will be measured in increased conductivity. I'm not sure about Redwood bark, but Oak (Quercus) bark definitely reduces pH and adds tannic substances. I've not tried it, but a fairly thick layer of <"Oak leaf litter, acorns cups and twigs"> should replicate the water conditions.If I want to breed blackwater species but I don't want to use peat, is it just the low conductivity that matters, or do I need to care about how many proton donors I'm adding to the water? For example, if I add tannins with a more neutral botanical like coir, should I also add a weak organic acid? Should I consider more acidic botanicals like redwood bark?
In really soft water the pH can easily be reduced by adding weak acids, ex-member @regani used <"citric acid">, most will use peat, Alder (Alnus spp.) "cones" etc.If I start with more alkaline water, I can observe that peat acidifies that water much more effectively than other botanicals (alder cones, leaves of various kinds). Indeed, many aquarists who've bred blackwater species have used RO water and acidify with peat.
Sphagnum peat works via <"ion exchange">, <"active substrates"> (that reduce pH) work in the same way.
I'd guess you would be better with RO, you can remove the hydroxide ion (OH-) by adding a proton (H+) but you still have the sodium ion (Na+) left. Because I use rain water I've never had to deal with the NaOH issue.Assume that I will start with RO water and remineralize. I probably don't want to start with my tap, which is soft, but contains NaOH.
cheers Darrel
- Messages
- 648
- Location
- San Francisco
Yes, I remember @regani actually used HCl with citric acid due to the short half life of the latter. I probably won’t do that. The half life of acetic acid, however, is quite a bit longer (~26 days). That said, I wonder if it’s the acid that’s important vs other substances in peat. Probably both.In really soft water the pH can easily be reduced by adding weak acids, ex-member @regani used <"citric acid">, most will use peat, Alder (Alnus spp.) "cones" etc.
I do use alder cones, but they’re not as potent as peat.
My thought is that the RO unit will exclude the Na+ and bring the OH- ions along to balance the charge. I know that it removes NaCl effectively.'d guess you would be better with RO, you can remove the hydroxide ion (OH-) by adding a proton (H+) but you still have the sodium ion (Na+) left. Because I use rain water I've never had to deal with the NaOH issue.
That said, in adddition:No, you just have to bear in mind that pH is more and more unstable as you approach pure H2O. Natural black water will remain acidic. A floating plant (or tree in the flooded forest) would have less effect on pH, because the oxygen evolved will mainly enter the atmosphere, rather than into the water.
Humic substances are in practice acting as a low pH-buffering system. The pH usually stabilizes at between 4.5 and 6.5 in a well maintained softwater tank run with RO or rainwater. Maintenance means regularly adding more botanicals and/or leaf litter or replacing depleted peat and whichever you use: waterchanges.
Peat is if you look at it concenttrated botanicals and mulm, accumulated over centuries and saturated with humic substances.I do use alder cones, but they’re not as potent as peat.
The RO unit will remove NaOH as it does NaCl.My thought is that the RO unit will exclude the Na+ and bring the OH- ions along to balance the charge. I know that it removes NaCl effectively.
- Messages
- 648
- Location
- San Francisco
Yes, the convenient thing about peat is that I can pour water over it in a funnel like coffee, so I can treat and measure the water before adding to the aquarium. I’m thinking of trying something similar with coir or shredded redwood, possibly adding acid if necessary.Peat is if you look at it concenttrated botanicals and mulm, accumulated over centuries and saturated with humic substances
Of course I also add botanicals to the tank, but I don’t rely on them to drive the chemistry of the water.
Yes, it's easy to extract. My personal problem with peat is that it's not sustainable. Most commercially available peat is taken from deposits that are cut largely unregulated, setting free lots of CO2 that had been stored in it for centuries.Yes, the convenient thing about peat is that I can pour water over it in a funnel like coffee, so I can treat and measure the water before adding to the aquarium. I’m thinking of trying something similar with coir or shredded redwood, possibly adding acid if necessary.
There are so many (weak) acids in peat and botanicals, why add additional acids? How acidic do you want it? Unless you want to get down to let's say a pH of 3.5 very quickly you don't need additional acids. For anything between 4.5 and 6 you can ommit using extra chemicals.
- Messages
- 648
- Location
- San Francisco
Here was my question above:Yes, it's easy to extract. My personal problem with peat is that it's not sustainable. Most commercially available peat is taken from deposits that are cut largely unregulated, setting free lots of CO2 that had been stored in it for centuries.
There are so many (weak) acids in peat and botanicals, why add additional acids? How acidic do you want it? Unless you want to get down to let's say a pH of 3.5 very quickly you don't need additional acids. For anything between 4.5 and 6 you can ommit using extra chemicals.
If I want to breed blackwater species but I don't want to use peat, is it just the low conductivity that matters, or do I need to care about how many proton donors I'm adding to the water? For example, if I add tannins with a more neutral botanical like coir, should I also add a weak organic acid? Should I consider more acidic botanicals like redwood bark?
- Messages
- 648
- Location
- San Francisco
@MacZ Sorry for the confusion. There's a lot going on in this thread. I'm interested in replicating the ease of extraction and repeatability of peat without using peat. For example, coir is supposedly a neutral botanical, so if I filter RO with coir, I might not get down to pH 4.5. My question is: is the acidity itself important, and if so, is there a difference between adding acid vs using an acidic botanical. I don't know if this is known, and I might just need to try it.
- Messages
- 2,868
- Location
- Wiltshire UK
Hi all,
cheers Darrel
That was just for your tap water, RO should be pretty near ion free.My thought is that the RO unit will exclude the Na+ and bring the OH- ions along to balance the charge. I know that it removes NaCl effectively.
cheers Darrel
Ah. The acidity in congruence with low conductivity makes for a very bacteria unfriendly environment. Which is necessary for the fishes health and breeding success.My question is: is the acidity itself important, and if so, is there a difference between adding acid vs using an acidic botanical. I don't know if this is known, and I might just need to try it.
Parallely without the humic substances the pH is unstable.
So humic substances are a must. If a species needs a certain very low acidity, this can be achieved by additional acid, of course.
Even with peat it is not re-peat-able (sorry...I'm interested in replicating the ease of extraction and repeatability of peat without using peat.
I only know how that works theoretically, I wouldn't dare trying. Besides the fact that in my country you can't just get a bottle of any acid.
- Messages
- 648
- Location
- San Francisco
Of course. What I mean is that within the same batch, the protocol is repeatable within 90%. But if I change the batch, I can adjust the protocol so that I know what the water is before adding it to the tank. If I rely only on botanicals added to the tank, I get a much larger change in the chemistry of the tank with each water change, and far less control.Even with peat it is not re-peat-able (sorry...) closer than 90%. It is almost impossible to achieve exactly the same conditions every time. As per above you can then drop further down using acids, but you will have to test and match every time you use them.
I only know how that works theoretically, I wouldn't dare trying. Besides the fact that in my country you can't just get a bottle of any acid.
I don't need glacial acetic acid for this, I think I would just try white vinegar. If it works (coir and/or redwood bark + vinegar) it's worth knowing that I can do this without peat, no?
Can't confirm that for tanks working on RO. Tap, yes. But once tank running on RO has found its balance I has no changes at all usually. I just add the roughly same amount of botanical extracts and it keeps working.I get a much larger change in the chemistry of the tank with each water change, and far less control.
Try anorganic acids. Not vinegar, it contains a lot of things besides the acid, which makes it even less controlable.I don't need glacial acetic acid for this, I think I would just try white vinegar. If it works (coir and/or redwood bark + vinegar) it's worth knowing that I can do this without peat, no?
- Messages
- 648
- Location
- San Francisco
I think white vinegar is just glacial + water, at 4% - 7% v/v.Try anorganic acids. Not vinegar, it contains a lot of things besides the acid, which makes it even less controlable.
- Messages
- 648
- Location
- San Francisco
Yeah, maybe it would be easier to just make a concentrated extract and add that each time. I’ll try that also.I just add the roughly same amount of botanical extracts and it keeps working.
As more than once mentioned: Peat is a natural product, so results may vary from batch to batch. It might, but you will need a lot, which can cause acute oxygen depletion. You first have to "neutralize" the KH, before anything is going to happen to the pH.(a) that lower the ph enough for their eggs to hatch
Depends. You will of course have to pretreat the water as you have to work against the KH you add with each waterchange and you don't want to do this in the tank.(b) will that make it a nightmare to do water changes.
I'll repeat it one more time:
First soften the water by dilution with RO (or use some type of purified water from the start), then add a proton donor. This is the safest and least messy way. Always first counteracting the KH is a constant factor for setbacks and errors.
Ok. Was 'friad of that; guess i'll have to wait till next year when i can install an ro unit (moving; can't install an ro unit at current residence).As more than once mentioned: Peat is a natural product, so results may vary from batch to batch. It might, but you will need a lot, which can cause acute oxygen depletion. You first have to "neutralize" the KH, before anything is going to happen to the pH.
Depends. You will of course have to pretreat the water as you have to work against the KH you add with each waterchange and you don't want to do this in the tank.
I'll repeat it one more time:
First soften the water by dilution with RO (or use some type of purified water from the start), then add a proton donor. This is the safest and least messy way. Always first counteracting the KH is a constant factor for setbacks and errors.
- Messages
- 2,868
- Location
- Wiltshire UK
Hi all,
This is how my little experiment went. It took a while (probably 45 minutes in all), even without calibrating the conductivity meter, (because the DI water read ~2 microS, I knew that the meter was pretty much spot on).
Most of the time was waiting for the pH meter to stabilise, I calibrated it (with 2 point calibration) both yesterday and this morning. I tested the conductivity before the pH (to avoid any possible contamination with the 3 mol. KCl storage solution). The only exception was the "leaves added" sample which had multiple pH and conductivity readings taken.
The meters are both relatively cheap ones. The Jenway 570 pH meter and the Hanna HI9535 Conductivity meter are ~£250 - £300 each.
Sorry there are lots of pictures, but roughly in order they are "DI water", "pH calibration", "Rain water" and "Rain water plus leaves".
Conductivity, DI water = 1.86 microS

pH, pH 4 buffer calibration

Conductivity, rainwater = 76 microS.

pH, rainwater = pH 7.7
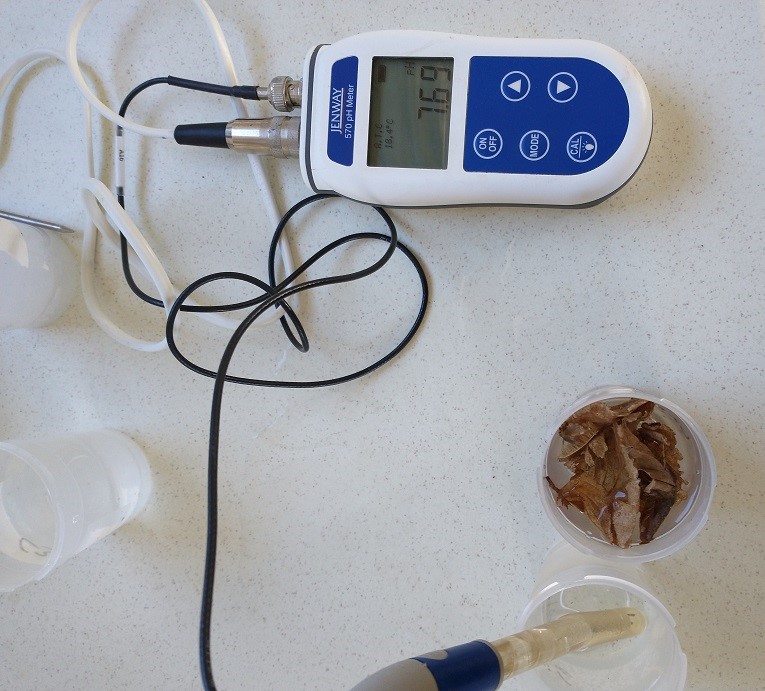
pH, leaves added = pH7.4

Conductivity, leaves left over night = 91 microS

pH, leaves overnight = pH 5.7

cheers Darrel
I used freshly collected dead <"Hornbeam (Carpinus betulus) leaves">, (there are Hornbeam hedges on the campus car park) partially because I was too lazy to go and pick some Oak (Quercus robur) leaves.(Rain water) ... at the moment it is about 50 microS, but still with a pH value around pH 8. (I'll get some photographs tomorrow when the light is better). If I add some Oak leaves the pH will fall
This is how my little experiment went. It took a while (probably 45 minutes in all), even without calibrating the conductivity meter, (because the DI water read ~2 microS, I knew that the meter was pretty much spot on).
Most of the time was waiting for the pH meter to stabilise, I calibrated it (with 2 point calibration) both yesterday and this morning. I tested the conductivity before the pH (to avoid any possible contamination with the 3 mol. KCl storage solution). The only exception was the "leaves added" sample which had multiple pH and conductivity readings taken.
The meters are both relatively cheap ones. The Jenway 570 pH meter and the Hanna HI9535 Conductivity meter are ~£250 - £300 each.
Sorry there are lots of pictures, but roughly in order they are "DI water", "pH calibration", "Rain water" and "Rain water plus leaves".
Conductivity, DI water = 1.86 microS
pH, pH 4 buffer calibration
Conductivity, rainwater = 76 microS.
pH, rainwater = pH 7.7
pH, leaves added = pH7.4
Conductivity, leaves left over night = 91 microS
pH, leaves overnight = pH 5.7
cheers Darrel
Attachments
Similar threads
- Replies
- 7
- Views
- 2K
- Replies
- 107
- Views
- 7K
Forum statistics
Latest profile posts
I'm looking for quality apistogrammas, can anyone recommend a good seller specialized in apistogrammas who ships in Europe? Thanks
