- Messages
- 2,871
- Location
- Wiltshire UK
Hi all,
Mike is right, the fish have evolved in water which have virtually no dissolved salts, and these are often the conditions black water fish need to breed successfully.
The relationship between compounds in the water and conductivity is shown by this graph, you can substitute any salts for NaCl, and for weak acids, like the humic acids from Indian almond leaves, peat or alder cones we can assume it will be the linear section of the HSO4 graph.

Whilst conductivity tells you how much salts you have, it doesn't tell you what they are. This is where pH comes in, the pH is a measure of the ratio of OH- ions and H+ ions (H + H + O = H2O) (or other acids and bases expressed as OH- and H+ ions). The easiest way to think of it is that acids are H+ donors and add H+ ions, and alkalis are H+ acceptors and remove H+ ions.
At the ratio of 1:1 H+:OH- the pH is pH7 (really it should be pH-7 (10-7 H+ ions)), more H+ ions are added and pH falls (more H+ added, at pH6 H+ = 10-6). Because we are dealing with powers to the base10, pH is a base10 log scale and pH5 is 100 times more acid than pH7, and pH4 1000 times.
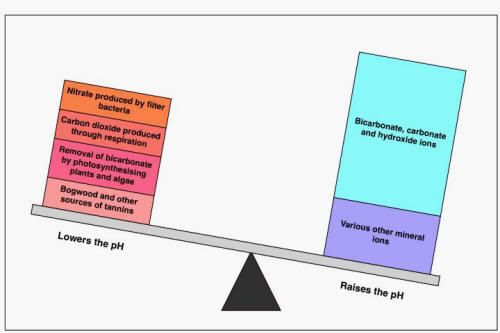
So pH tells us the ratio, but not the amount. If you think of scales that are in balance, this is pH7. That balance could be that we have 10Kg in either scale or 10g, pH doesn't differentiate. If we add 10g of "acid" to the 10kg scale, we will have changed the balance very, slightly to the acid side, and the pH will fall, but only slightly to the high pH6 range. We can think of the 10Kg in the alkali as our "buffering". If we add the same amount (10g) of acid to our 10g scale, we now a ratio of 2:1 acid:alkali and our pH will drop much further and much faster.
This is the scales for well buffered alkaline water, but it shows the principle.
cheers Darrel
Mike is right, the fish have evolved in water which have virtually no dissolved salts, and these are often the conditions black water fish need to breed successfully.
I'll have a go at this, pure H2O is an electrical insulator, what we call "water" is actually almost always a dilute solution of salts. This is why adding compounds doesn't help, it raises the TDS. We can add enough acid to counteract any amount of alkalinity, but we are getting further away from H2O all the time. We can only get more suitable water by removing (RO water) or exchanging (Ca2+ etc. for 2H+ in humic compounds) ions.Also, if you dont mind, i am not quite sure I understand conductivity and how it relates to fish health? I understand pH, hardness, and obviously temperature, but what are the ramifications of too high or too low conductivity?
The relationship between compounds in the water and conductivity is shown by this graph, you can substitute any salts for NaCl, and for weak acids, like the humic acids from Indian almond leaves, peat or alder cones we can assume it will be the linear section of the HSO4 graph.

Whilst conductivity tells you how much salts you have, it doesn't tell you what they are. This is where pH comes in, the pH is a measure of the ratio of OH- ions and H+ ions (H + H + O = H2O) (or other acids and bases expressed as OH- and H+ ions). The easiest way to think of it is that acids are H+ donors and add H+ ions, and alkalis are H+ acceptors and remove H+ ions.
At the ratio of 1:1 H+:OH- the pH is pH7 (really it should be pH-7 (10-7 H+ ions)), more H+ ions are added and pH falls (more H+ added, at pH6 H+ = 10-6). Because we are dealing with powers to the base10, pH is a base10 log scale and pH5 is 100 times more acid than pH7, and pH4 1000 times.
So pH tells us the ratio, but not the amount. If you think of scales that are in balance, this is pH7. That balance could be that we have 10Kg in either scale or 10g, pH doesn't differentiate. If we add 10g of "acid" to the 10kg scale, we will have changed the balance very, slightly to the acid side, and the pH will fall, but only slightly to the high pH6 range. We can think of the 10Kg in the alkali as our "buffering". If we add the same amount (10g) of acid to our 10g scale, we now a ratio of 2:1 acid:alkali and our pH will drop much further and much faster.
This is the scales for well buffered alkaline water, but it shows the principle.

cheers Darrel